Energy Content Of Food
Energy content of food. Biology Lab Report Doctor Emad Mohammad Mari fPurpose. 1 the components of food that provide energy protein fat carbohydrate alcohol polyols organic acids and novel compounds should be determined by appropriate analytical methods. Food energy is defined as the energy released from carbohydrates fats proteins and other organic compounds.
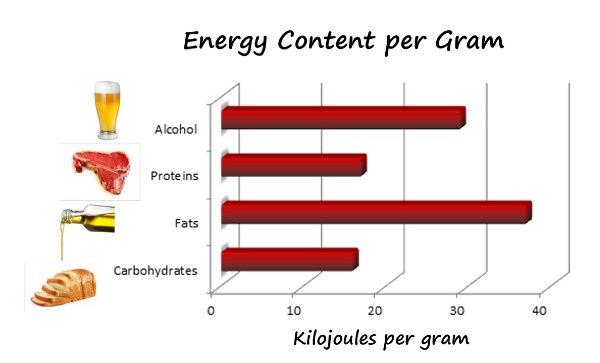
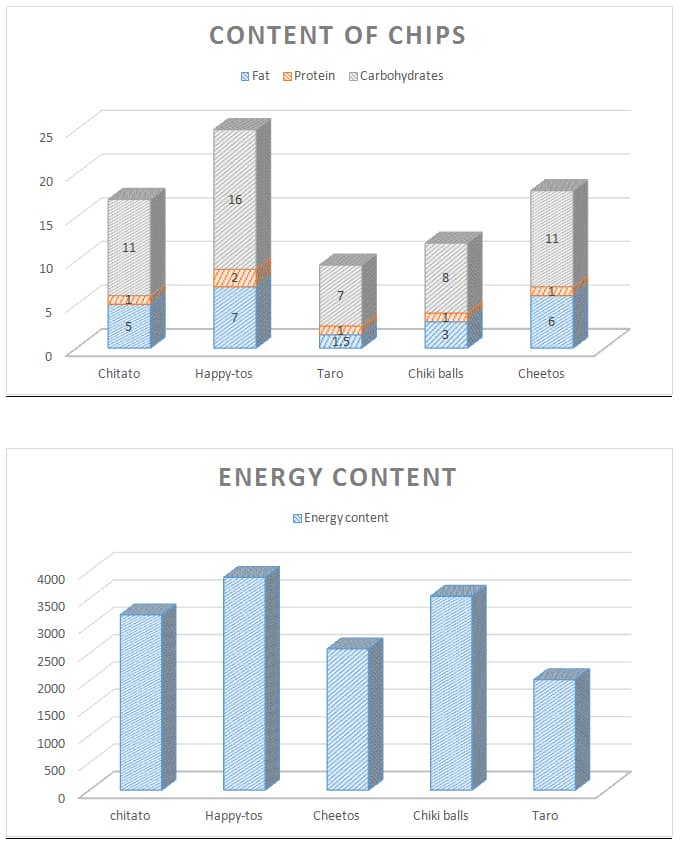
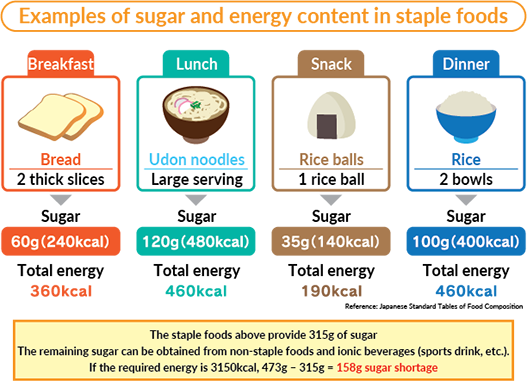
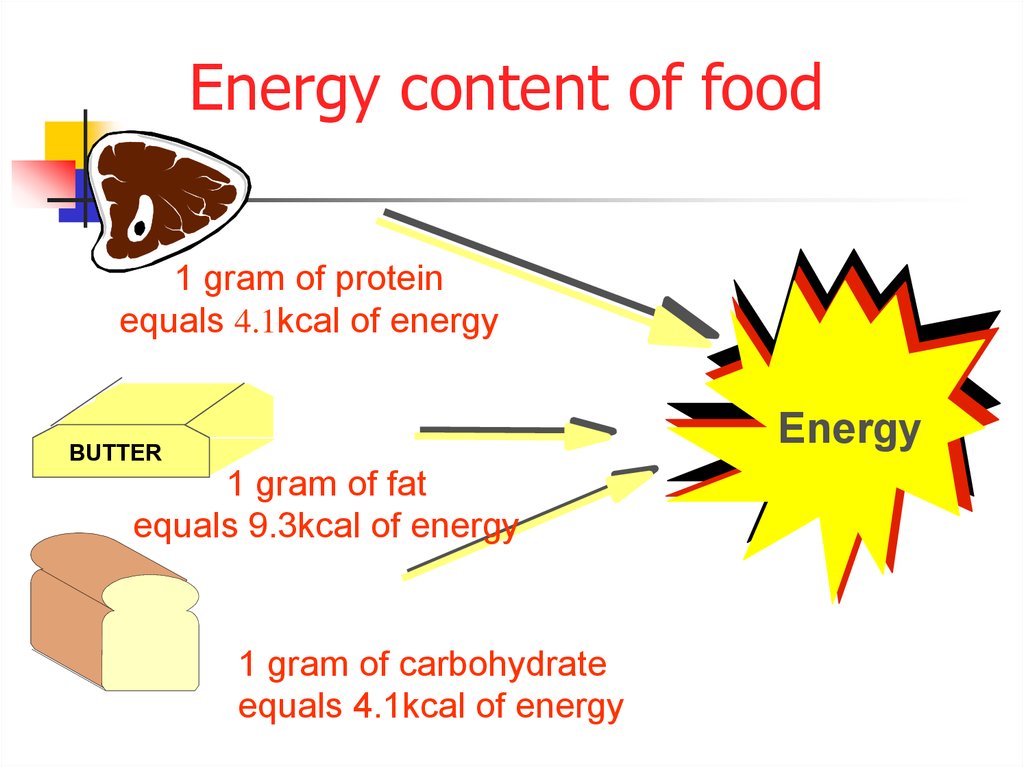
When the three major calorigenic nutrients carbohydrates fats and proteins in a food are burnt entirely with sufficient amounts of oxygen it releases energy or food calories that are expressed in kilojoules kJ or kilocalories kcal. How much energy they provide depends on the amount of carbohydrate sugarsstarch protein fat and alcohol the food or drink contains as well as the portion size. Hypothesis Foods depending on their carbohydratefat composition have different energy content that can.
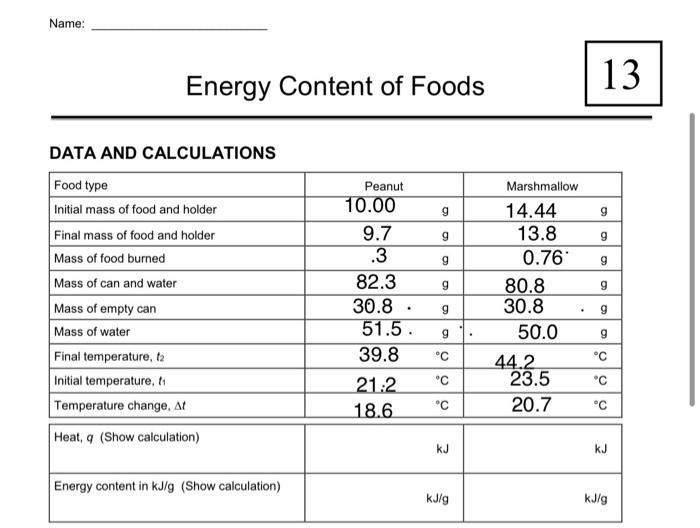
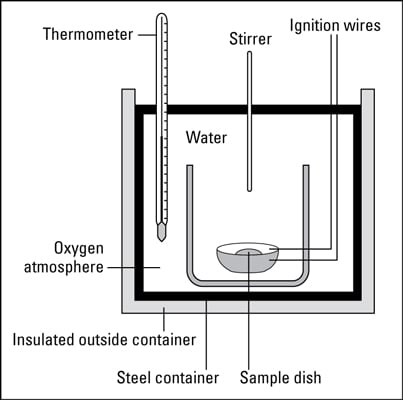
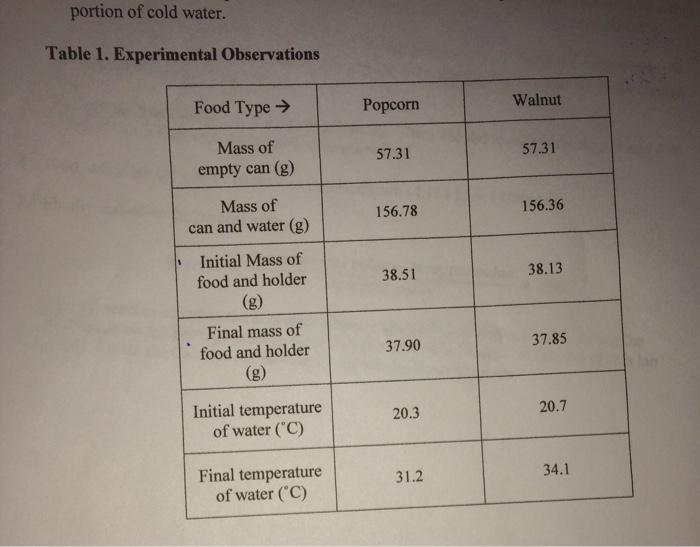
Energy content is the amount of heat produced by the burning of 1 gram of a substance and is measured in joules per gram Jg. Record the mass of the food sample. Adapted from Food energy - methods of analysis and conversion factors.
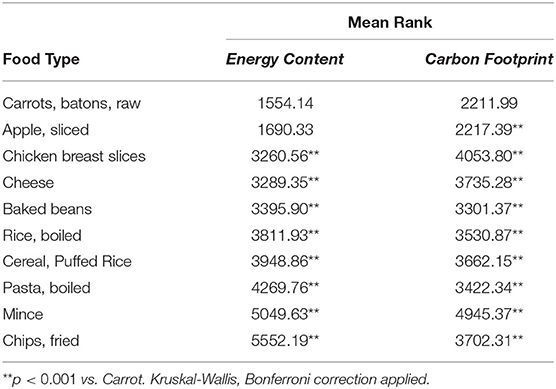
Measuring the Heat of Combustion of Food. This is a good place to point out that what we commonly call calories in food are really kilocalories kcal on the food packaging 1 kilocalorie 1000 calories. Samples released 18 MJkg the highest of the food groups and the average energy output for all foods was 1431 MJkg.
The carbohydrate samples contained the least energy with 1263 MJkg. For classes familar with the equation q m CT where q is the heat energy m the mass of water heated C the specific heat of water 42 J g 1 deg 1 and ΔT the. You will look for patterns in the amounts of energy released during burning of the different foods.
Different ingredients in food and how they are prepared mean some have more kilojoules than others. Basically we all require energy in order to live. We oxidize and digest the food and in the process release and.
Other smaller components of the diet such as organic acids polyols and. Record the starting temperature of the water.
Energy released J mass of water g x rise in temperature C x 42.
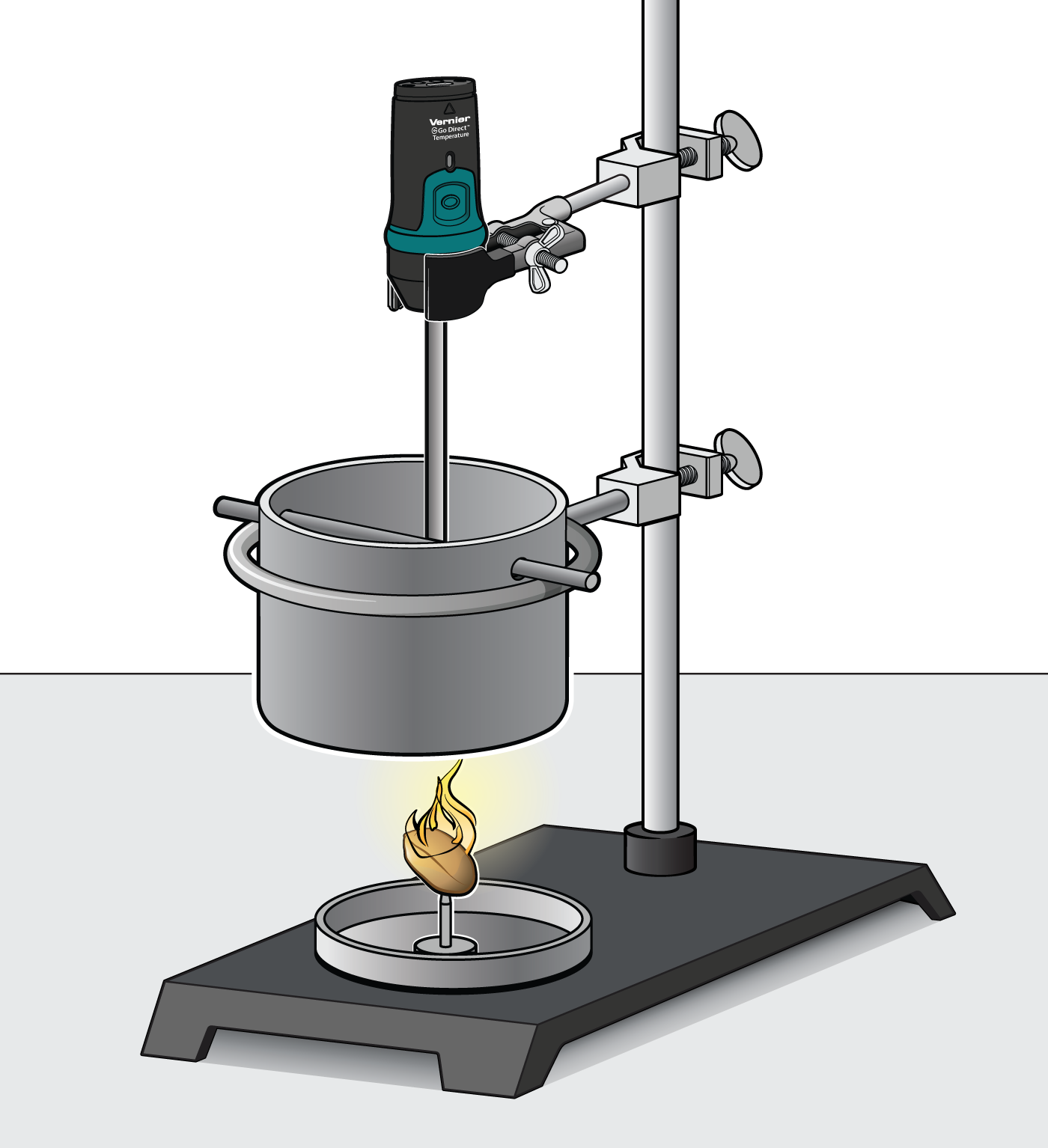
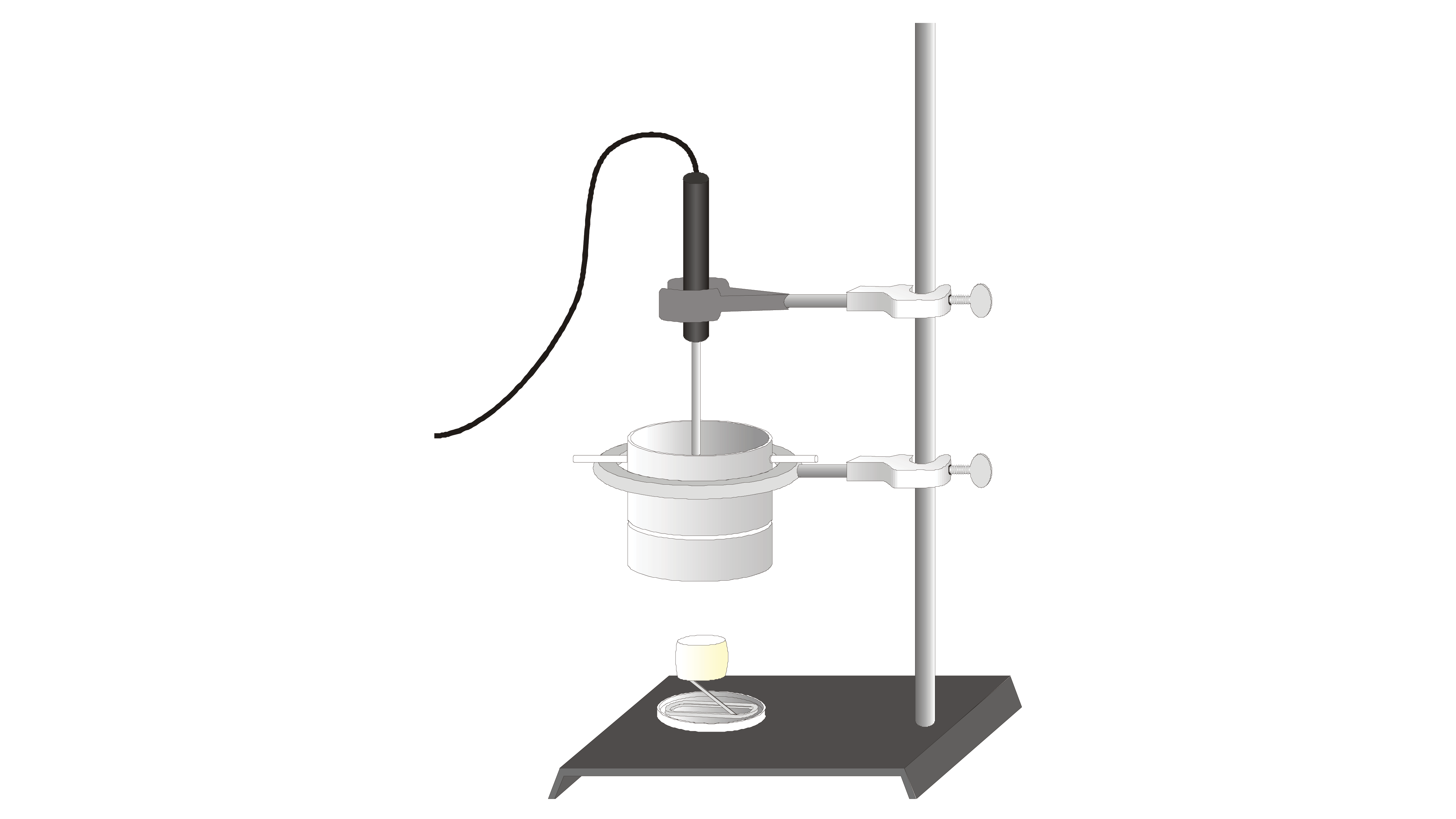
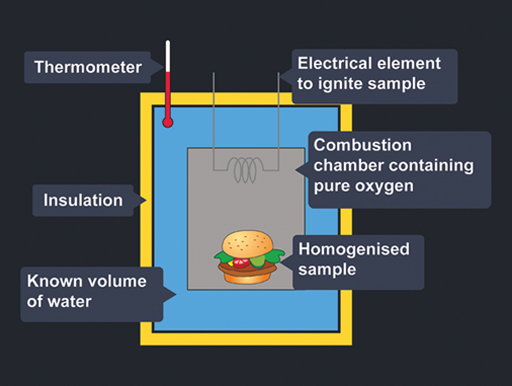
If we to trace the food back it takes us to photosynthesis a process by which green plants capture the suns energy transforming it into chemical energy that is stored in the chemical structures of molecules. The kCalories the Body Expends Components of Energy Expenditure Thermic effect of food TEF is estimated at 10 of total energy intake and involves digestion and absorption. Pour water into a boiling tube. You will look for patterns in the amounts of energy released during burning of the different foods. This is a good place to point out that what we commonly call calories in food are really kilocalories kcal on the food packaging 1 kilocalorie 1000 calories. Calculate the energy value of a food. You can determine energy content by burning a portion of food and capturing the. The purpose of this experiment is to investigate different foods energy content by observing how much and how long 4 different types of nuts will take to make a liquid water temperature rise until the water reaches boiling point or the nut. Basically we all require energy in order to live.
Energy Content of Food - YouTube. Record the mass of the food sample. Food energy is chemical energy that animals derive from their food to sustain their metabolism including their muscular activity. Determining the energy content of foods depends on the following. If we to trace the food back it takes us to photosynthesis a process by which green plants capture the suns energy transforming it into chemical energy that is stored in the chemical structures of molecules. Other smaller components of the diet such as organic acids polyols and. This is a good place to point out that what we commonly call calories in food are really kilocalories kcal on the food packaging 1 kilocalorie 1000 calories.
































Post a Comment for "Energy Content Of Food"